With hormone imbalances becoming more prevalent in modern-day society, many practitioners are desiring to learn how to best support their client’s hormonal health.
For many practitioners and hormone specialists, advising clients to avoid exposure to endocrine-disrupting chemicals (EDCs) is an easy and effective way to promote hormone health and balance. However, do we ever stop to wonder why this approach is so effective? Or why it even needs to be considered in the first place?
Although the importance of avoiding EDCs is a common conversation within the women’s health field, there is much more to understand about how exactly these compounds impact the body and endocrine system, and why our exposure to them is so prevalent.
In this article, we’re going to take the conversation around avoiding EDCs a step deeper by discussing the reason why EDCs are so proliferative in our society, and the underlying physiological impact that these compounds can have on the body, especially in regard to hormone health.

What is the Endocrine System?
The endocrine system is made up of various organs and glands responsible for receiving and secreting hormonal messages in response to certain stimuli. To put it simply, the endocrine system is the body’s primary line of communication.
When the brain experiences an internal or external stimulus, endocrine glands within the brain (Hypothalamus, Pituitary, and Pineal glands) produce hormones that travel through the bloodstream to reach other endocrine glands within the body such as the Adrenals, Thyroid, and Gonads. After receiving their hormonal cues, these endocrine glands produce another set of hormones that enter the bloodstream. These hormones eventually arrive at the intended organs to deliver messages that instruct specific actions. The result of those actions usually yields either a positive or negative feedback response which then travels back to the brain to inform the hypothalamus of the completed or uncompleted task.
These feedback responses comprise what are called the Hypothalamic-Pituitary Axes. The primary HP axes are the HPA (Hypothalamic-Pituitary-Adrenal) axis, the HPT (Hypothalamic-Pituitary-Thyroid) axis, and the HPG (Hypothalamic-Pituitary-Gonadal) axis. Although these axes work independently to target specific actions of certain tissues and glands, they can also impact each other.
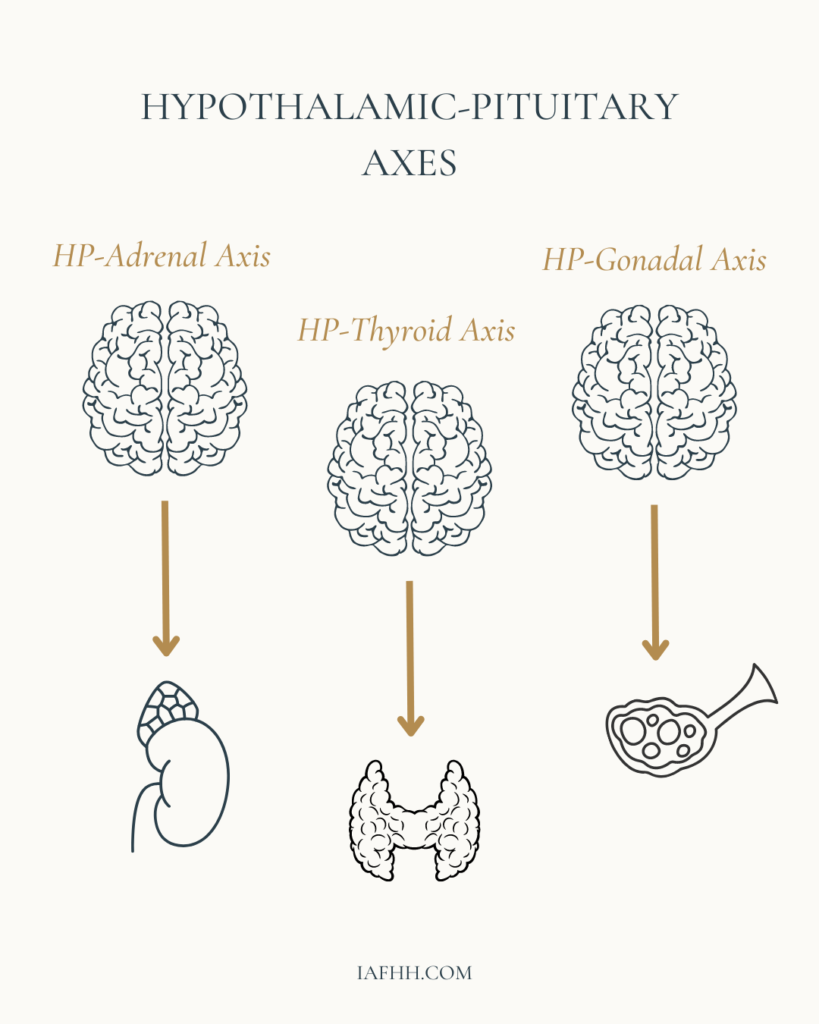
As a whole, the entire endocrine system is quite intricate and very specific. It works hard to maintain a level of homeostasis within the body. However, when certain disruptions occur within these hypothalamic-pituitary axes, it can lead to the development of hormone imbalances. This can result in the common “hormonal” symptoms our female clients may face such as PMS, heavy/painful bleeds, weight gain, breakouts, and/or fertility struggles.
Due to the impact that the endocrine system can have on the entire body, disruptions to the endocrine system may also negatively influence areas outside of reproductive health such as digestive health, blood sugar regulation, mental stability, and stress modulation.
Some of the most common disruptors that can interfere with the function of the endocrine system are endocrine-disrupting chemicals.

What Are Endocrine-Disrupting Chemicals?
Endocrine-disrupting chemicals (EDCs) — sometimes referred to as hormone disruptors or xenoestrogens — consist of various chemicals and compounds that have exogenous hormone properties. These compounds have the ability to mimic, interfere with, or block the function of endogenous hormones such as estrogen, progesterone, and thyroid hormone. Some EDCs are found naturally within nature, such as phytoestrogens and mycoestrogens. However, the vast majority of these disruptive compounds are found in man-made synthetic chemicals and products that contain them.
These disruptive compounds are capable of negatively impacting not only reproductive health but a person’s overall health and well-being. The unfortunate thing is that for modern society, EDCs are all around us, all of the time.
EDCs can often be found in common household items such as cleaning products, dish/hand soap, shampoo/conditioner, lotion, makeup, and perfume, as well as in the food we eat, the water we drink, and even in the air that we breathe. Another common area where EDCs can hide is within conventional brands of menstrual hygiene and period products such as pads, tampons, feminine washes, douches, and lubricants. Finally, one of the most disruptive EDCs of them all is hormonal birth control, specifically combined contraceptives that contain both ethinylestradiol and progestin.
Although exposure to some EDCs may not be easily escaped, those found in common household and beauty care items can be quickly recognized on product and food labels, and either avoided or replaced with healthier, non-toxic alternatives.
Common Endocrine-Disrupting Compounds:
- Phthalates (Parfum, Fragrance)
- Parabens (Methylparaben, Propylparaben, Benzylparaben, Butylparaben, Ethylparaben, Isobutylparaben)
- Glycol Ethers (2-Butoxyethanol, Methoxydiglycol, Propylene Glycol)
- Bisphenols (BPA, BPS, BPF, Phenol)
- Octinoxates (Parsol, 2-Ethylhexyl, P-Methoxycinnamate)
- Nitrosamines (DEA, TEA)
- Preservatives (BHA, BHT, Formaldehyde)
- Teflon (Polytetrafluoroethylene)
- Pesticides (Glyphosphate, Atrazine, DDT)
- Others (Triclosan, Dioxin, Perchlorate, Benzene, Benzophenone, Homosalate, PABA, Polyvinyl Chloride, Ethinylestradiol, Progestin)

The convenience of Endocrine-Disrupting Chemicals
Over time, modern society has grown to develop an unhealthy dependence on the use of convenience items that contain these endocrine disruptive chemicals. Although many of these products may be affordable and easily accessible, their “convenience” comes at a great cost.
Since the early-mid 1900s, we have been aware of the impact that endocrine disrupting chemicals can have on the world, particularly in regard to their impact on the reproductive function of wildlife, as well as their cancer-causing effects in both humans and animals. [1]
In the 1940s – 1960s, researcher Rachel Carson discovered the impact that synthetic pesticides, specifically DDT, had on wildlife and humankind. Through observing the accumulative impact of DDT on animals, Carson discovered that it may contain DNA-damaging and cancer-causing risks, which she wrote about in her book titled “Silent Spring”. [2]
Whereas, in the book “Our Stolen Future”, researchers discovered that endocrine disruptive compounds found in many household and beauty products may have an impact on future generations of human health due to the influence these chemicals can have on fetal growth and reproductive development in exposed pregnant individuals. [3]
In addition, the World Health Organization (WHO) indicated in their World Health Statistics 2014 Report that there has been a noticeable worldwide decline in human and wildlife reproductive function likely due to the increase in EDC usage and exposure over time. [4]
Although these synthetic chemicals are often cheaper to produce and package as everyday products, and synthetic pesticides may be more effective at eradicating undesired pests than are more natural alternatives, their convenience may very well have an impact on the health and development of humankind for years to come.
With respect to the environment and the livelihood of future generations, we really should be questioning whether or not the “convenience” is actually worth it.

The Physiological Impact of Endocrine-Disrupting Chemicals
Many are aware that EDCs can negatively impact hormone health, but how exactly are they able to interfere with such a tightly-regulated and homeostatic-oriented system such as the endocrine system, and what impact might they have on human health beyond reproduction?
In many settings, exogenous hormones, such as xenoestrogens and other endocrine-disrupting compounds can interfere with hormone health by disrupting the production and utilization of endogenous hormones and their receptor sites, as well as interfering with proper hormone metabolism and elimination. Altogether this can set the stage for imbalances such as estrogen excess and/or dominance, progesterone deficiency, and thyroid disorders.
EDC impact on hormone production
Some research shows that certain EDCs such as perchlorate may interfere with thyroid hormone synthesis by inhibiting the uptake and utilization of iodide in thyroid cells, resulting in impaired thyroid hormone production and an increased risk for hypothyroidism. [5] This is not only problematic for hormone health and balance, but thyroid health is also imperative for maintaining effective energy levels, metabolism, and healthy weight maintenance, all of which may be impaired through the presence of EDC-induced hypothyroidism. In addition, EDCs, particularly xenoestrogens (synthetic exogenous estrogens), such as those found in Phthalates have been shown to interfere with androgen production in fetal and neonatal male rats. [6], [7]
Other forms of exogenous hormones, such as those found in synthetic estrogen medications such as hormonal birth control and hormone replacement therapy, may impact hormone production by interfering with the positive and negative feedback loops of the hypothalamic-pituitary axes. [8] In turn, this may result in impaired production of endogenous hormones such as FSH, LH, estrogen, and progesterone.
EDC impact on hormone utilization
Within nearly every cell of the body are receptor sites for hormones. This is one of the ways that our body is able to utilize hormones and the messages they carry. Various EDCs have been shown to interfere with cellular hormone receptors by either activating or inhibiting their function. [1], [9]
Regarding xenoestrogens and their interference with estrogen receptor function, particularly concerning their proliferative effects, excessive exposure may increase one’s risk for the development of estrogen-related growths and cancers. [9], [10]
In addition, certain EDCs that are capable of displacing endogenous hormones at their receptor sites and/or from their binding proteins, such as sex hormone binding globulin (SHBG) or thyroxine-binding globulin (TBG) may increase the circulation of free and unbound hormones, and impair proper tissue uptake and cellular utilization of endogenous hormones. [11], [12]
EDC impact on hormone elimination
When it comes to endogenous hormones, specifically estrogen, the body tends to want to use them and lose them. Excess levels of circulating estrogens within the body can pose risk for many complications such as excessive endometrial and breast tissue proliferation, gallbladder disease, and thyroid disorders.
Naturally, to prevent certain hormone imbalances such as estrogen dominance from occurring, the body has protective mechanisms to help keep estrogen levels in check. The majority of estrogen metabolism occurs within the liver through Phase I and Phase II detoxification. Learn more about estrogen excess and metabolism.
Unfortunately, EDCs, specifically PCBs and PAHs can impair the metabolism and clearance of estrogen through both Phase I and Phase II liver detoxification, increasing the production of catechol estrogen metabolites, and posing risk for DNA damage, and other estrogen excess-related complications. [13], [14]
Furthermore, EDCs, specifically BPA and ethinylestradiol may interfere with the Phase III metabolism of estrogen within the colon, increasing the presence of pathogenic bacteria and beta-glucuronidase activity in rodents. [15] In turn, this may increase the presence of recirculating estrogens, further impacting one’s estrogen balance.

The Risk of Endocrine-Disrupting Chemicals
Although hormones are most often considered in regard to one’s fertility and fecundity, hormone health has an impact in many areas beyond reproductive health. Learn more about the importance of hormone health beyond fecundity.
By interfering with metabolic function and increasing the risk of diabetes, obesity, and other complications [1], as well as increasing the risk of hormone-responsive growths and cancers, EDCs can influence a person’s health, well-being, and quality of life in a myriad of ways.
Although it may be challenging to completely avoid all exposure to EDCs, through awareness and education we can certainly take measures to help our clients recognize where the most common EDCs may be lurking in their homes, and advocate for them to purchase and use more natural, non-toxic alternatives.
When supporting your clients in making the switch to more natural alternatives, here are a few resources and tools you can share with them:
Looking for More Resources?
Want more tools and strategies for supporting your client’s hormone health so you can maximize your ability to support them in all areas of their life? Be sure to check out our practitioner training, the IAFHH Functional Hormone Specialist Certification Program.
By receiving the cutting-edge research and specialized education available in our program, you will quickly excel in your private practice and become the go-to hormone health expert in your community. In this program, we support you with all the tools, resources & information you need to expand your expertise in functional hormone health, get your female clients to achieve real & lasting results, and excel in your women’s health practice.

About the Author
Ashe Milkovic, NTP, IC-FHS, FBCS
Ashe is the founder and CEO of The International Association for Functional Hormone Health. She has a passion for spreading awareness about the power of functional nutrition for optimizing hormones & fertility, and her mission is to build community and safe space for other practitioners and aspiring learners to expand their knowledge and expertise in functional hormone health.
Article Sources
- Paterni I, Granchi C, Minutolo F. Risks and benefits related to alimentary exposure to xenoestrogens. Crit Rev Food Sci Nutr. 2017 Nov 2;57(16):3384-3404. doi: 10.1080/10408398.2015.1126547. PMID: 26744831; PMCID: PMC6104637.
- Carson, R., Wilson, E. O., Lear, L. J., Darling, L., & Darling, L. (2022). Silent spring. Mariner Books.
- Colborn, T., Dumanoski, D., & Myers, J. P. (1997). Our stolen future. Abacus.
- Mita, D. G. (2016). Editorial: Environment and health: The problem of the endocrine disruptors. The Open Biotechnology Journal, 10(1), 10–12. https://doi.org/10.2174/1874070701610010010
- Wolff J. Perchlorate and the thyroid gland. Pharmacol Rev. 1998 Mar;50(1):89-105. PMID: 9549759.
- Louise G. Parks, Joe S. Ostby, Christy R. Lambright, Barbara D. Abbott, Gary R. Klinefelter, Norman J. Barlow, L. Earl Gray, Jr. The Plasticizer Diethylhexyl Phthalate Induces Malformations by Decreasing Fetal Testosterone Synthesis during Sexual Differentiation in the Male Rat. Toxicological Sciences, Volume 58, Issue 2, December 2000, Pages 339–349, https://doi.org/10.1093/toxsci/58.2.339
- Mylchreest, E., Sar, M., Wallace, D. G., & Foster, P. M. D. (2002). Fetal testosterone insufficiency and abnormal proliferation of leydig cells and gonocytes in rats exposed to di(n-butyl) phthalate. Reproductive Toxicology, 16(1), 19–28. https://doi.org/10.1016/s0890-6238(01)00201-5
- Spona, J., Feichtinger, W., Kindermann, C., Wünsch, C., & Brill, K. (1996). Inhibition of ovulation by an oral contraceptive containing 100 μg levonorgestrel in combination with 20 μg ethinylestradiol. Contraception, 54(5), 299–304. https://doi.org/10.1016/s0010-7824(96)00183-7
- Shanle EK, Xu W. Endocrine disrupting chemicals targeting estrogen receptor signaling: identification and mechanisms of action. Chem Res Toxicol. 2011 Jan 14;24(1):6-19. doi: 10.1021/tx100231n. Epub 2010 Nov 5. PMID: 21053929; PMCID: PMC3119362.
- Wang X, Ha D, Yoshitake R, Chan YS, Sadava D, Chen S. Exploring the Biological Activity and Mechanism of Xenoestrogens and Phytoestrogens in Cancers: Emerging Methods and Concepts. Int J Mol Sci. 2021 Aug 16;22(16):8798. doi: 10.3390/ijms22168798. PMID: 34445499; PMCID: PMC8395949.
- Huixiao Hong, William S. Branham, Hui Wen Ng, Carrie L. Moland, Stacey L. Dial, Hong Fang, Roger Perkins, Daniel Sheehan, Weida Tong. Human Sex Hormone-Binding Globulin Binding Affinities of 125 Structurally Diverse Chemicals and Comparison with Their Binding to Androgen Receptor, Estrogen Receptor, and α-Fetoprotein. Toxicological Sciences, Volume 143, Issue 2, February 2015, Pages 333–348, https://doi.org/10.1093/toxsci/kfu231
- Déchaud H, Ravard C, Claustrat F, de la Perrière AB, Pugeat M. Xenoestrogen interaction with human sex hormone-binding globulin (hSHBG). Steroids. 1999 May;64(5):328-34. doi: 10.1016/s0039-128x(98)00114-7. PMID: 10406482.
- Retrieved: http://aacrjournals.org/cebp/article-pdf/11/12/1560/2261737/ce1202001560.pdf
- Kester MH, Bulduk S, Tibboel D, Meinl W, Glatt H. Falany CN, Coughtrie MW, Bergman A, Safe SH, Kuiper GG, Schuur AG, Brouwer A, Visser TJ. Potent inhibition of estrogen sulfotransferase by hydroxylated PCB metabolites: a novel pathway explaining the estrogenic activity of PCBs. Endocrinology. 2000 May;141(5):1897-900. doi: 10.1210/endo.141.5.7530. PMID: 10803601.
- Javurek AB, Spollen WG, Johnson SA, Bivens NJ, Bromert KH, Givan SA, Rosenfeld CS. Effects of exposure to bisphenol A and ethinyl estradiol on the gut microbiota of parents and their offspring in a rodent model. Gut Microbes. 2016 Nov;7(6):471-485. doi: 10.1080/19490976.2016.1234657. Epub 2016 Sep 13. PMID: 27624382; PMCID: PMC5103659.
